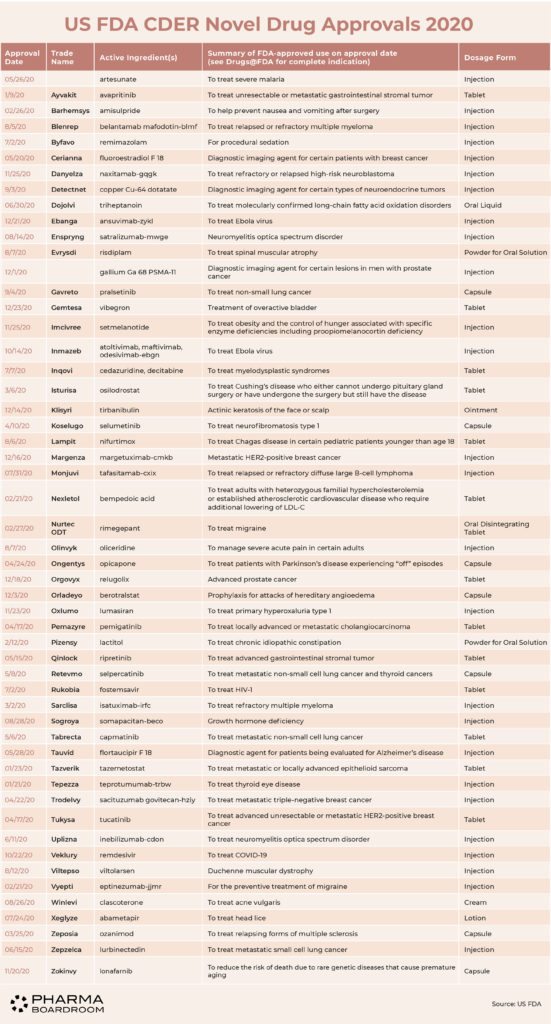
In 2020, the US FDA's Center for Drug Evaluation & Research (CDER) approved 53 novel drugs, up from 48 in 2019, across a range of therapeutic areas, including the first treatments for COVID-19 patients.
 The FDA's Center for Biologics Evaluation & Research (CBER) is charged with approving new biologic therapies, eight of which were approved in 2020.
The FDA's Center for Biologics Evaluation & Research (CBER) is charged with approving new biologic therapies, eight of which were approved in 2020.
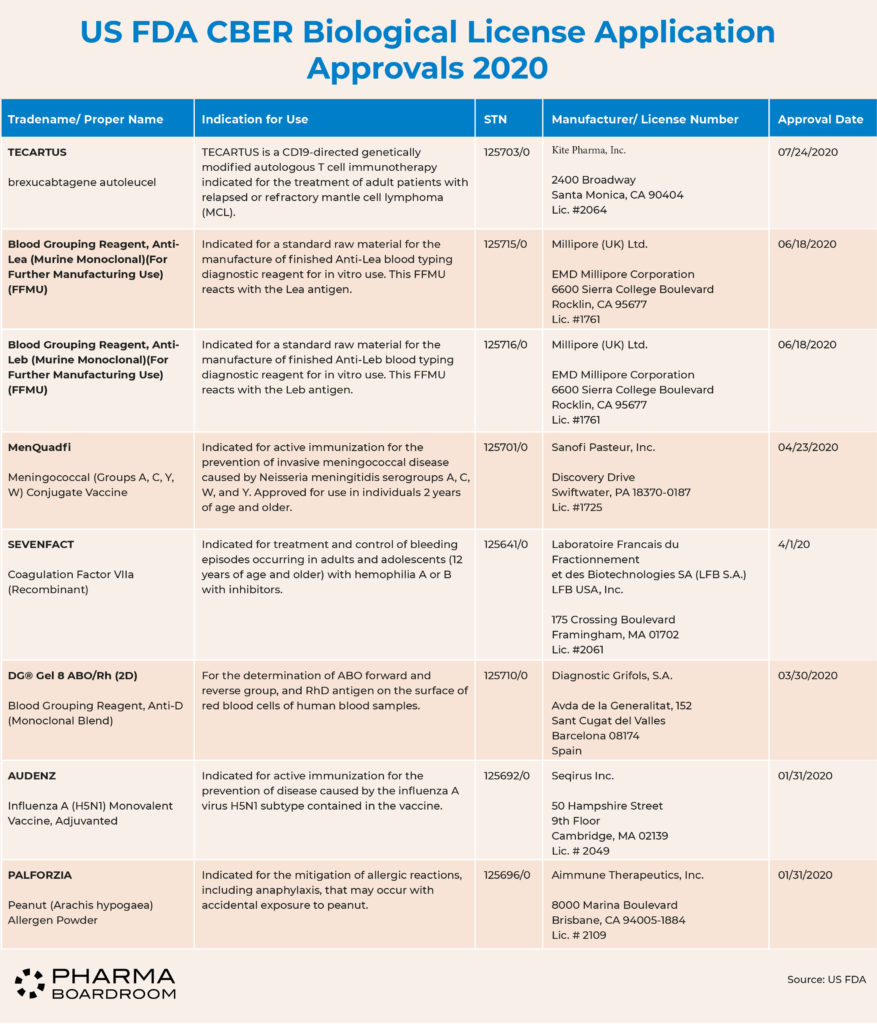
 The FDA's Center for Biologics Evaluation & Research (CBER) is charged with approving new biologic therapies, eight of which were approved in 2020.
The FDA's Center for Biologics Evaluation & Research (CBER) is charged with approving new biologic therapies, eight of which were approved in 2020.







